
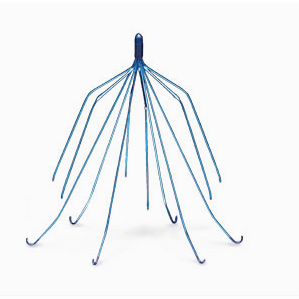
How Long Should Retrievable Filters be left in Place? (Rex Medical) Argon Medical OptionELITE IVC Filter.Our attorneys are reviewing potential lawsuits for people who were injured after receiving the following types of IVC filters: Constant and Severe Pain in the Heart, Chest or Elsewhere in the Body.Hematoma or Nerve Injury at the Puncture Site.Perforation, Puncture or Serious Damage to the Heart, Lungs or Vena Cava.Trauma patients are being implanted with IVC filters at increasing rates despite a lack of proof that the devices help, the researchers concluded. They found that not only did the IVC filter recipients fail to receive a survival benefit, but they also suffered deep vein thrombosis (DVT) events at an increased rate. For the study, researchers looked at 803 patients who received an IVC filter after a traumatic event between 20. The researchers noted this was a “significantly higher rate” of perforation.Īnother study published in the Annals of Surgery in October 2015 found that patients who suffer traumatic injuries face a “life-threatening” risk for blood clots, but that IVC filters don’t provide a survival benefit. In June 2015, a study published in the Journal of Vascular Interventional Radiology found that the Cook Celect IVC filter had a 43% rate of perforation compared to the Option filer made by Rex Medical, which had a 0% perforation rate. 1 patient had a blood clot inside the filter.2 patients’ filters had migrated to a position that wouldn’t allow for removal, and.3 patients couldn’t have their devices removed because the filters had punctured a blood vessel.8 patients couldn’t have their filters removed because they were embedded.Of the 13 unsuccessful IVC filter removal attempts: Doctors made 13 unsuccessful removal attempts, 11 of which occurred in patients whose filters had remained in place for over 85 days. About 680 patients were implanted with retrievable vena cava filters, and only 58 were successfully removed. IVC Filter StudiesĪn April 2013 study published in JAMA Internal Medicine looked at complications associated with IVC filter placement in 952 patients. Some of these reports led to adverse complications in patients, and may have been related the devices being left implanted for long periods of time, beyond the time when the blood clot risk has passed, according to the FDA. 146 embolizations (detachment of device components).921 device adverse event reports involving IVC filters.At the time of the warning, the agency had received at least: In August 2010, FDA issued a Safety Communication regarding adverse events associated with retrievable IVC filters. Food & Drug Administration (FDA) has received thousands of adverse reports associated with retrievable IVC filters. The devices intercept clots in the bloodstream and, over time, the clots break down. Gform.Retrievable inferior vena cava (IVC) filters are implanted into the veins of patients who are unable to take blood thinner medications in order to prevent blood clots from traveling to the lungs (pulmonary embolism). Δ document.getElementById( "ak_js_2" ).setAttribute( "value", ( new Date() ).getTime() ) We tell you about cash you can claim every week!ĬAPTCHANameThis field is for validation purposes and should be left unchanged. District Court for the Eastern District of Missouri. The Blood Clot Filter Side Effects Lawsuit is Case No 4:16-cv-01116 in the U.S.
#Class actio suit on heart blood clot filters free
An IVC filter lawsuit may help you obtain compensation for your medical bills, pain and suffering, lost wages and more. Submit your information now for a FREE case evaluation. If you or a loved one suffered IVC filter side effects such as migration, IVC perforation, DVT or pulmonary embolism, you may have a legal claim. They bring claims of defective manufacturing, design defect, defect due to inadequate warning, negligence, failure to recall, failure to warn, breaches of express and implied warranty and negligent representation and fraud among others. Many of the plaintiffs in this blood clot filter side effects lawsuit suffered failed removals and ongoing injuries as a result of Bard’s devices. Allegedly, Bard received clearance for their new device without adequate testimony on the safety of it. They claim that because Bard targeted bariatric, trauma, cancer and orthopedic patients who were only temporarily at risk, Bard tripled its sales.Īlso, because physicians were eager to remove the devices once the risks subsided for their patients, Bard produced a retrievable device and sold it to physicians.


 0 kommentar(er)
0 kommentar(er)
